Optical microscopy
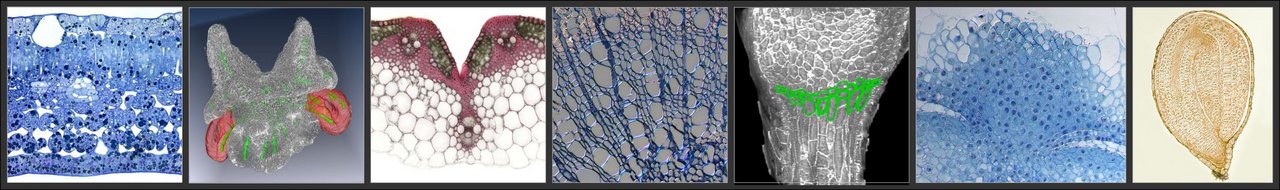
Conventional light microscopy (resolution limit of about 200 nm) is used primarily to visualise cell anatomy in preparations of fresh or fixed tissue sections embedded in resin, following its staining using a variety of either histological or fluorescent dyes. Three dimensional models of tissues or organs can be assembled by combining the data retrieved from a series of sections.
Digital microscopy
Digital microscopy enables live material (whole plants, individual organs or tissues) to be visualised by altering the plane of focus of the instrument. The resolution here is 260-500 nm. Effectively this approach addresses the gap between stereomicroscopy and scanning electron microscopy.
Confocal microscopy
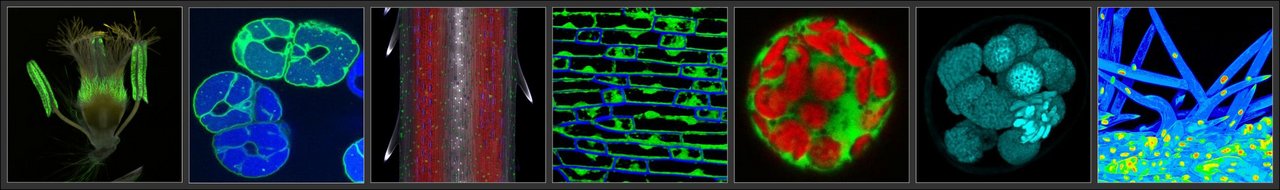
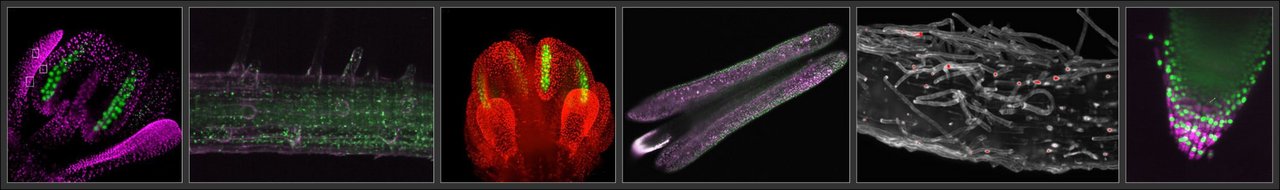
Confocal microscopy (resolution limit of 180-500 nm) facilitates the optical sectioning of either fixed or fresh specimens, which, for example, allows for the localization of either organelles which autofluoresce or fluorescently labelled molecules. The three-dimensional structure of specific organs or tissues can be reconstructed from Z-stacked images.
Light Sheet Fluorescence Microscopy (LSFM)
Light Sheet Fluorescence Microscopy(LSFM) is particularly suited for imaging of living, and also for imaging of large specimens at high spatial and temporal resolution. Samples are illuminated with a thin sheet of light allowing optical sectioning, keeping out-of-focus areas in darkness hence minimizing photodamage. For live-cell imaging, plant samples are mounted in a nutrient-rich media, vertically, in a chamber equipped with light and temperature control, allowing samples to stay alive and be monitored for up to several days.
Super-resolution light microscopy
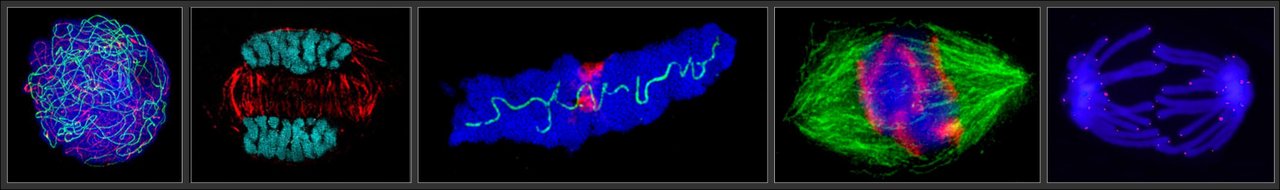
Super-resolution light microscopy (SRM) achieves a structural resolution of 20-100 nm through the combined use of structured illumination (SIM) and photo-activated localization microscopy (PALM). In contrast to confocal microscopy, even the smallest fluorescence-marked structures and multiple fluorescences can be found in a cell, cell nucleus or chromosome, can be visualized in high resolution.