Metabolische Diversität
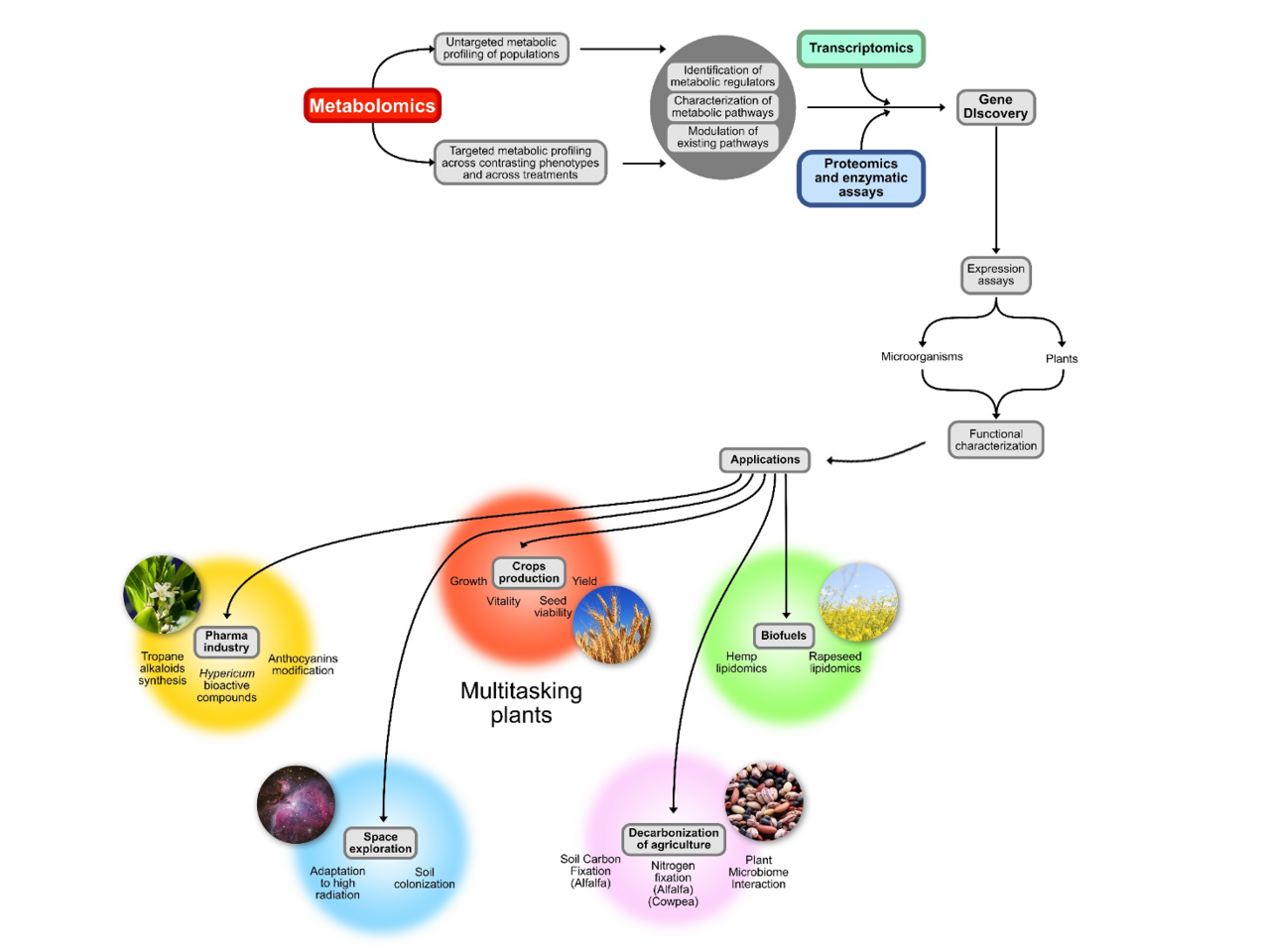
Das höchste Ziel der Gruppe Metabolische Diversität am IPK ist es, das Zusammenspiel zwischen Pflanzengenen, der Umwelt und den molekularen Resultaten dieser Interaktionen zu verstehen, um Pflanzeneigenschaften zu optimieren, die für die moderne Landwirtschaft von Nutzen sind. Wir definieren metabolische Diversität daher als die Fähigkeit von Pflanzen, ihre biosynthetischen Produkte anzupassen, wenn sie verschiedenen abiotischen und biotischen Faktoren in ihrer Umgebung ausgesetzt sind. Diese Fähigkeit, sich schnell auf Umweltreize einzustellen, ist extrem wichtig für neue Generationen von Kulturpflanzen und die Menschen, die von ihnen abhängig sind. Wir wissen jetzt, dass diese metabolische Vielfalt eine starke genetische Komponente hat.
Die erwarteten Ergebnisse der Arbeitsgruppe Metabolische Diversität reichen von zielgerichtet quantifizierten Metaboliten für spezifische Vergleiche essentieller primärer Biomoleküle bis hin zu breiten ungerichteten metabolischen Screens. Wir beabsichtigen, Unterschiede zwischen Populationen von Nutzpflanzen zu identifizieren, um landwirtschaftlich wichtige Merkmale zu selektieren. Die Gruppe stützt sich stark auf einen kombinatorischen experimentellen Ansatz, der Biochemie, Molekularbiologie und analytische Chemie integriert, um den primären und sekundären Stoffwechselstatus in Kombination mit phänotypischen Leistungsparametern (Wachstumsraten, Vitalität, Samenlebensfähigkeit und Ertrag) zu analysieren.
Neben Getreidepflanzen hat die Forschungsgruppe Interesse an medizinischen Nutzpflanzenarten und ist darüber hinaus daran interessiert, neuartige Techniken der synthetischen Biologie einzusetzen, um aus Pflanzen gewonnene medizinische Wirkstoffe in einer Vielzahl von alternativen Organismen zu produzieren. Schließlich will das Metabolische Diversität Labor einen Beitrag zur Lösung fundamentaler Probleme in den modernen Pflanzenwissenschaften leisten. Insbesondere beabsichtigen wir, einen signifikanten Beitrag zur Zuordnung von Genfunktionen in Form der Annotation von Enzymklassen und -aktivitäten für den hohen Anteil von Genen zu leisten, die als "mutmaßlich" oder, was noch wichtiger ist, mit "unbekannter Funktion" identifiziert wurden und in komplexen Genomen von Kulturpflanzen allgegenwärtig sind.
scroll top
Projekte
Plant Metabolism and Engineering – From Cars to Mars
A green solution to the production of valuable organic compounds would be a boon to the environment, the economy, and to the advancement of our knowledge of metabolic pathways. Plants can be a tremendous and valuable resource for the production of complex compounds that would normally require vast resources and involve environmentally damaging chemicals when produced by traditional methods. The D'Auria lab at the IPK in Gatersleben is interested in engineering value added traits into plants and micro-organisms to be solutions for problems ranging from biofuels to space exploration. Currently, the research team led by Dr. D'Auria is focused on the engineering of the tropane alkaloid biosynthetic pathway for use as pharmaceutical compound production. Several tropane alkaloids are listed by the World Health Organization as the most essential medicines needed for basic health care.
Other ongoing studies are focused on identification of gene controlling elements (promotors) involved in tissue specific expression. As an example, the figure on the right shows a plant construct where a seed specific promotor was identified. These control elements will be essential for targeted metabolic gene expression. In this case, genes encoding enzymes to modify seed oils for biofuels are targeted to the highly enriched oil producing seeds.
The D’Auria lab is also interested in functional metabolomics. As an example, we have identified key enzymes for modifying anthocyanins, the compounds responsible for the red to purple shades of colors in leaves and flowers. Anthocyanins are ingredients in ‘super foods' because of their anti-oxidant and cancer fighting qualities. The figure to the right depicts normal Arabidopsis plants while the plant to the right was engineered to not only over-produce anthocyanins, but to modify the actual normal end-product into accumulating an intermediate anthocyanin to high concentrations.
There are no petrochemicals in space. This one simple fact precludes making pharmaceuticals and other complex organic molecules on a manned mission to Mars or to the moon. Bringing up a pharmacopeia of relevant compounds would be weight prohibitive when there are better solutions. The D'Auria lab is interested in modifying the plants that would be grown in space for food and oxygen so that they would also produce the astronaut's medicines. The first steps are fully understanding the pathways involved in the process of biosynthesizing these essential medicinal compounds. The next steps will include re-engineering these pathways in plants and micro-organisms and optimizing their production. The D'Auria labs efforts are focused on understanding tropane alkaloid and hypericin/hyperforin production via multiple methods. These include MALDI imaging of where pharmaceuticals accumulate, protein crystallography and modeling of key biosynthetic enzymes as well as their localization via immunohistochemistry. Mixing and matching enzymes from different plant families wield broaden the possibilities for metabolic engineering of novel medicinal compounds.
Hypericum research – connecting transcriptomics to metabolomics
Hypericum perforatum, also known as Saint John’s Wort (SJW), is part of a genus including over 400 species distributed all around the world and characterized by great diversity (Crockett and Robson 2012). Saint John’s Wort is a popular medicinal plant that produces bio active compounds and is recognized for its mild antidepressant properties (Schallau et al 2010; Galla et al 2011; Rizzo 2016). Hypericin is one of the many bioactive compounds produced by SJW that got the attention of the scientific community thanks to its potential use in the treatment of the Alzheimer disease (Sgarbossa et al 2008; Bramanti et al 2010) and to its applications in cancer photodynamic therapy (Garg et al 2010, 2012; Dudek-Peric 2015). Despite the long effort in the study of hypericin production, this biosynthetic pathway remains mostly uncharacterized.
Dr. Paride Rizzo, a postdoc of the D’Auria lab, is interested in the developmental biology of SJW. His research lead to the identification of contrasting phenotypes of glanded and glandless tissues (G-/G+ PT) in the flowers of H. perforatum (Rizzo et al 2019).
We compare these phenotypes to identify the main metabolic and transcriptomic differences between glanded and glandless tissues. Using this combination of genetic and metabolic subtraction, we identify candidate genes for the biosynthesis of hypericin and for the differentiation of dark glands.
Currently the MD group is implementing a pipeline for the test of such genes using expression assays in microorganisms as well as in plants.
Considering the high metabolic cost of synthesizing hypericin and producing glands to keep it compartmentalized, we are curious about the ecological and evolutionary advantages of its biosynthesis. With this in mind, we explore the metabolic and genomic diversity of H. perforatum and combine the phylogenesis of this species with its hypericin production patterns in order to understand if the trait of dark glands formation has a geographical structure and if it is going to be lost in evolution.
While we characterize several genotypes of the species perforatum, we are also addressing the interspecific diversity of the genus Hypericum thanks to a germplasm collection that we are currently growing in our lab.
Our research is embedded in a network of partners including: IPB Halle, Halle University, University of Braunschweig, University of Saskatchewan and University of Heidelberg.
With our research on Hypericum we want to generate new scientific results that will be useful for the implementation of future applications in the pharmaceutical industry and in medical research.
Previous Studies
Tropane alkaloids represent a major class of plant-derived secondary metabolites known to occur in the Solanaceae family but are also present in the families Convolvulaceae, Proteaceae, Rhizophoraceae and Erythroxylaceae. The core defining structure of tropane alkaloids is an 8-azabicyclo[3.2.1] octane nucleus. The diversity of tropane alkaloids is achieved by elaboration of this core through different types of modifications. The genus Erythroxylum (family Erythroxylaceae) contains approximately 230 species with ranges spread throughout the tropics including South America and Madagascar.
Erythroxylum coca and Erythroxylum novogranatense are the most widely used species for the production of cocaine. Very little is known as to the biological and ecological roles that cocaine and other tropane alkaloids play in plants. Their anti-cholinergic properties argue strongly in favor of deterrent activity against herbivores. We have begun molecular and biochemical studies in order to elucidate the biochemical steps which lead to the production of tropane alkaloids in E. coca plants.
The terminal step in the production of cocaine or other tropane related esters is thought to be the formation of the acyl ester via the action of an acyltransferase enzyme. In the case of cocaine, this acyltransferase utilizes the substrates methylecgonine and benzoyl CoenzymeA to produce cocaine and free CoA. Our lab has been working for several years on a plant specific family of acyltransferases commonly referred to as the BAHD acyltransferases. Thus far, more than 8 BAHD acyltransferases have been isolated from Erythroxylum coca (E. coca). Recent results show one of these BAHD members exhibits cocaine synthase activity. Members of my group have successfully developed an LC-MS based ‘realtime' enzyme assay for cocaine synthase in order to obtain very accurate kinetic data for characterization studies. We are also using antibodies made against the whole purified protein in order to perform immunoprecipitation and immunohistochemical studies.
In addition to the study of the role of acyltransferases in E. coca, members of the D’Auria lab are also actively pursuing the enzymes that are involved in forming the first and second rings of the tropane core. Most theories to date suggest that the precursor compound is most likely the mono-methylated polyamine putrescine. Benjamin Chavez, a PhD. student in the D’Auria lab, is characterizing the properties of several polyamine synthases that are similar to putrescine methyltransferase and spermine/spermidine synthases. We are also interested in the origins of the benzoic acid portion of cocaine and are combining the tools that we have thus far developed for E. coca to develop this system as a model for benzoic acid biosynthesis.
Technologies and Resources
Equipment
Gas chromatography - mass spectrometry (GC-MS, Leco HT TOF-MS)
Using a combination of a LECO HT time-of-flight mass spectrometer, an AGILENT 7890A gas chromatograph equipped with a GERSTEL MPS2XL autosampler, we are able to quantify a large array of polar plant metabolites. Typically, these comprise a number of 60-100 known metabolites like amino acids, organic acids, polyamins, sugars, sugar phosphates etc. and twice the number of metabolites with unknown chemical structure. Routinely we apply in-line derivatization (derivatization of each individual sample prior injection) to improve the quality of our measurements. This enables us to quantify metabolites from large sample numbers in a highly reproducible manner.
High resolution liquid chromatography - mass spectrometry (LC-MS, BRUKER Maxis 2 QTOF-MS)
A high resolution Bruker Maxis 2 quadrupole time-of-flight mass spectrometer (R = 85000, accuracy = 0.6 ppm) hyphenated with an AGILENT 1290 liquid chromatograph (max 1200 bar) and equipped with a GERSTEL autosampler is used for high throughput metabolite profiling of semi-polar compounds like secondary metabolites, phytosterols, vitamins, hormones... and hydrophobic analytes like lipids, tocoperols, pigments... Typically, several Hundreds to Thousands non-redundant chromatographic features (mz) are quantitatively recorded which can be partially annotated by database queries (KNApSAcK, KEGG) using accurate mass, isotopic pattern and MSMS fragment spectra of the analytes. The liquid chromatograph can be coupled to a mikroplate fraction collector (ADVION Nanomate) to comprehensively isolate separated metabolites for in-depth characterization. The analysis time ranges from 5 min (secondary metabolite profiling, lipid profiles of oil storing organs) to 30 min (comprehensive profiling of semi-polar metabolites, lipid profiling of samples with complex lipidomic composition) and allows reproducible measurements of a large number of samples.
HyperSpEED
In the frame of horizon 2020, the societal challenge number 1: “Health demographic and wellbeing” is defined. Europe is experiencing steady aging of its workforce with consequent heavier weight put on the healthcare system. Some strategies being considered to face this challenge are the development of more personalized medicine and the development of green and sustainable solutions. The main goal of this strategy is to help the elderly to remain active and independent.
The scientific knowledge that will be gathered during the HyperSpEED project will address these points by filling big gaps that are currently limiting the pharmaceutical industry. We will achieve this by addressing the biodiversity of the genus Hypericum.
Saint John’s Wort (Hypericum perforatum L.) is a popular medicinal plant used for centuries in traditional medicine that came to the attention of modern plant biology because of its multiple applications in the treatment of depression, different types of cancer and Alzheimer’s disease. We are interested in understanding the biosynthetic processes that lead to the formation of bioactive compounds that are highly valuable for the pharma industry and that could provide relevant benefits for the aging population.
The genus Hypericum includes more than 460 species that are adapted to very different environments around the planet. Despite this rich diversity, the species that caught the majority of the scientific interest in previous studies is H. perforatum.
With the HyperSpEED project (Hypericum multi-Species Exploration of Extracts Diversity), we intend to explore the interspecific diversity of this genus to identify new accessions valuable for potential uses in medical applications. This will be achieved by using a combination of transcriptomics, metabolomics, and genomics. These methods will produce novel information on gene expression patterns associated with the synthesis of molecules of high pharmaceutical value. Furthermore, we will continue our research on the species H. perforatum for which several genes involved in the biosynthesis of hypericin, hyperforin, and other pharmaceutically important compounds, will be characterized.
This research is embedded in the broader concept of “multitasking plants for a sustainable future” pursued by the Metabolic Diversity Group. The project is embedded in a consortium entirely based in Sachsen-Anhalt and connected to a network of partners in, Saskatoon (Canada), Oslo (Norway), Braunschweig and Hannover.
The scientific results produced in the frame of HyperSpEED will be made available for future research following the principle of data reusability.
Representative Publications
- "Tropinone biosynthesis requires catalysis by a non-canonical type III polyketide synthase followed by P450-mediated ring closure." Bedewitz MA, Jones AD, D'Auria JC, Barry CS (2018). Nature Communications 9 (1), 5281.
- "Efficient synthesis of (S)-4-(1-methylpyrrolidin-2-yl)-3-oxobutanoate as the key intermediate for tropane alkaloid biosynthesis without racemization."Katakam NK, Seifert CW, D'Auria JC, Li G. (2018). Heterocycles (99).
- "Improved synthesis of N-methylcadaverine.",Anderson K, Moaven S, Unruh DK, Cozzolino AF, D'Auria JC.(2018) Molecules (23) E1216.
- "Tropane and granatane alkaloid biosynthesis: A Systematic analysis." Kim, N, Estrada, O, Chavez B, Stewart C, D'Auria JC (2016). Molecules 21(11), 1510-1535. doi:10.3390/molecules 21111510.
- "The last step in cocaine biosynthesis is catalyzed by a BAHD acyltransferase."Schmidt, G., Jirschitzka, J., Porta, T., Reichelt, M., Luck, K., Pardo-Torre, J., Dolke, F., Varesio, E., Hopfgartner, G., Gershenzon, J., D'Auria, J. (2015).Plant Physiology, 16(1), 89-101. doi:10.1104/pp.114.248187.
- "Influence of medium and elicitors on the production of cocaine, amino acids and phytohormones by Erythroxylum coca calli.",Docimo T, Davis AJ, Luck K, Fellenberg C, Reichelt M, Phillips M, Gershenzon J, D'Auria JC.(2015) Plant Cell, Tissue and Organ Culture (PCTOC)doi: 10.1007/s11240-014-0660-8.
- "Increasing the Pace of New Discoveries in Tropane Alkaloid Biosynthesis". Jan Jirschitzka, Franziska Dolke, John C. D'Auria,. In Nathalie Giglioli-Guivarc'h, editors : Advances In Botanical Research, Vol. 68,Burlington: Academic Press, 2013, pp. 39-72. ISBN: 978-0-12-408061-4.
- "Selection and validation of reference genes for quantitative gene expression studies in Erythroxylum coca", Docimo, T., Schmidt, G., Luck, K., Delaney, S. K., D'Auria, J (2013).Faculty of 1000 Research,2:37. doi:10.3410/f1000research.2-37.v1.
- "Learning from nature: new approaches to the metabolic engineering of plant defense pathways", Jirschitzka, J., Mattern, D. J.,Gershenzon, J., D'Auria, J. (2013).Current Opinion in Biotechnology,24(2),320-328. doi:10.1016/j.copbio.2012.10.014.
- "The biosynthesis of hydroxycinnamoyl quinate esters and their role in the storage of cocaine in Erythroxylum coca." Pardo-Torre, J., Schmidt,G., Paetz, C., Reichelt, M., Schneider, B., Gershenzon, J.' D'Auria, J. (2013). Phytochemistry, 91, 177-186. doi:10.1016/j.phytochem.2012.09.009.
- "The first step in the biosynthesis of cocaine in Erythroxylum coca: the characterization of arginine and ornithine decarboxylases.", Docimo, T., Reichelt, M., Schneider, B., Kunert, G., Gershenzon, J., D'Auria, J. ( 2012).Plant Molecular Biology, 78(6), 599-615. doi:10.1007/s11103-012-9886-1.
- "Plant tropane alkaloid biosynthesis evolved independently in the Solanaceae and Erythroxylaceae.", Jirschitzka, J., Schmidt, G., Reichelt, M., Schneider, B., Gershenzon, J., D'Auria, J. (2012). Proceedings of the National Academy of Sciences of the United States of America, 109(26), 10304-10309. doi:10.1073/pnas.1200473109.
- "Contribution of CoA ligases to benzenoid biosynthesis in Petunia flowers. ", Klempien, A.; Kaminaga, Y.; Qualley, A.; Nagegowda, D. A., Widhalm, J. R., Orlova, I., Shasany, A. K., Taguchi, G., Kish, C. M., Cooper, B. R., D'Auria, J., Rhodes, D., Pichersky, E., Dudareva, N. (2012). The Plant Cell, 24,2014-2030. doi:10.1105/tpc.112.097519.
- "Evaluation of candidate reference genes for real-time quantitative PCR of plant samples using purified cDNA as template.", Phillips, M., D'Auria, J., Luck, K.; Gershenzon, J. (2009).Plant Molecular Biology Reporter, 27(3), 407-416. doi:10.1007/s11105-008-0072-1.
- "The Arabidopsis thaliana type I isopentenyl diphosphate isomerases are targeted to multiple subcellular compartments and have overlapping functions in isoprenoid biosynthesis.", Phillips, M. A.; D'Auria, J., Gershenzon, J., Pichersky, E. (2008) The Plant Cell, 20(3), 677-696. doi:10.1105/tpc.107.053926.
- "Characterization of a BAHD acyltransferase responsible for producing the green leaf volatile (Z)-3-hexen-1-yl acetate in Arabidopsis thaliana." D'Auria, J., Pichersky, E.,Schaub, A., Hansel, A., Gershenzon, J. (2007).The Plant Journal, 49(2), 194-207.
- "Identification and characterization of the BAHD acyltransferase malonyl CoA: Anthocyanidin 5-O-glucoside-6''-O-malonyltransferase (At5MAT) in Arabidopsis thaliana."D'Auria, J., Reichelt, M., Luck, K., Svatos, A., Gershenzon, J. (2007).FEBS Letters, 581, 872-878.
- "Characterization of seed-specific benzoyloxyglucosinolate mutations in Arabidopsis thaliana." Kliebenstein, D. J., D'Auria, J., Behere, A. S., Kim, J. H., Gunderson, K. L., Breen, J. N., Lee, G., Gershenzon, J., Last, R. L., Jander, G. (2007).The Plant Journal, 51(6), 1062-1076.
- "Acyltransferases in plants: a good time to be BAHD." D'Auria, J. (2006). Current Opinion in Plant Biology, 9(3), 331-340.
- "The secondary metabolism of Arabidopsis thaliana: growing like a weed." D'Auria, J., Gershenzon, J. (2005).Current Opinion in Plant Biology, 8(3), 308-316.
- "An Arabidopsis thaliana gene for methylsalicylate biosynthesis, identified by a biochemical genomics approach, has a role in defense." Chen, F., D'Auria, J., Tholl, D., Ross, J. R., Gershenzon, J., Noel, J. P., Pichersky, E. (2003). An Arabidopsis thaliana gene for methylsalicylate biosynthesis, identified by a biochemical genomics approach, has a role in defense. The Plant Journal, 36(5), 577-588. Retrieved from ://000186532400001.
- "Biosynthesis and emission of terpenoid volatiles from Arabidopsis flowers." Chen, F., Tholl, D., D'Auria, J., Farooq, A., Pichersky, E., Gershenzon, J. (2003). Biosynthesis and emission of terpenoid volatiles from Arabidopsis flowers. The Plant Cell, 15(2), 481-494. Retrieved from ://000181008700014.
scroll top
Mitarbeitende
scroll top
Publikationen
Frohn S, Haas F B, Chavez B G, Dreyer B H, Reiss E V, Ziplys A, Weichert H, Hiltemann S, Ugalde J M, Meyer A J, DAuria J C, Rensing S A, Schippers J H M:
Evolutionary conserved and divergent responses to copper zinc superoxide dismutase inhibition in plants. Plant Cell Environ. (2026) Epub ahead of print. https://dx.doi.org/10.1111/pce.15198
Ye W, Leite Dias S, Mamin M, Arce C C M, Turlings T C J:
Three closely related Spodoptera species similarly affect gene expression and phytohormone levels but differentially induce volatile emissions in maize. Plant Cell Environ. (2026) Epub ahead of print. https://dx.doi.org/10.1111/pce.70389
Brendel F L:
Characterization and isolation of genes and enzymes involved in gramine biosynthesis in Lupinus species. (Master Thesis) Halle/S., Martin-Luther-Universität Halle-Wittenberg (2025) 27 pp.
Carréra J C, Guerra-Guimarães L, D’Auria J C, de Jesus Sartori L, Pinheiro C, Silva V A, Volpato M L, Carvalho G R, Mori F A:
Non-targeted metabolomic analysis of field-grown Coffea arabica cultivars reveals distinct leaf metabolic signatures. Theor. Exp. Plant Physiol. 37 (2025) 27. https://dx.doi.org/10.1007/s40626-025-00373-4
Chavez B G:
Unraveling topane alkaloid biosynthesis in Erythroxylum coca. (PhD Thesis) Halle/S., Martin-Luther-Universität Halle-Wittenberg, Naturwissenschaftliche Fakultät I Biowissenschaften (2025) 127 pp.
Knoch D, Rugen N, Thiel J, Heuermann M C, Kuhlmann M, Rizzo P, Meyer R C, Wagner S, Schippers J H M, Braun H-P, Altmann T:
A spatio-temporal transcriptomic and proteomic dataset of developing Brassica napus seeds. Sci. Data 12 (2025) 759. https://dx.doi.org/10.1038/s41597-025-05115-4
Leite Dias S:
Exploring plant allelopathy: from alkaloid pathway evolution to enzyme discovery and crop engineering. (PhD Thesis, kumulativ) Jena, Friedrich-Schiller-Universität Jena, Fakultät für Biowissenschaften (2025) 266 pp.
Leite Dias S, DAuria J C:
The bitter truth: how insects cope with toxic plant alkaloids. J. Exp. Bot. 76 (2025) 5-15. https://dx.doi.org/10.1093/jxb/erae312
Leite Dias S, Rizzo P, DAuria J C, Kochevenko A:
Efficient Agrobacterium-mediated methods for transient and stable transformation in common and Tartary buckwheat. Int. J. Mol. Sci. 26 (2025) 4425. https://dx.doi.org/10.3390/ijms26094425
Reincke A:
The role of N-methyltransferases in the biosynthesis of gramine in plants. (Bachelor Thesis) Köthen, Hochschule Anhalt, Studiengang Biotechnologie (2025) 71 pp.
Schüler D, Lange M, Altmann T, Cuacos M, Arend D, D’Auria J C, Fiebig A, Kumlehn J, Neumann K, Melzer M, Rey-Mazón E, Rolletschek H, Scholz U, Willner E, Reif J C:
Data management in balance – a decade of balancing pragmatism, sustainability and innovation at plant research center IPK Gatersleben. J. Integr. Bioinform. 22 (2025) 20250012. https://dx.doi.org/10.1515/jib-2025-0012
Spanic V, Duvnjak J, Hefer D, DAuria J C:
Changes in metabolites produced in wheat plants against water-deficit stress. Plants 14 (2025) 10. https://dx.doi.org/10.3390/plants14010010
Wonneberger R, DAuria J C, Neumann K, Hansen P B, Dieseth J A, Nielsen L K, Niemelä T, Odilbekov F, Novakazi F, Bengtsson T, CResWheat Consortium:
Integrating metabolomics and high-throughput phenotyping to elucidate metabolic and phenotypic responses to early-season drought stress in Nordic spring wheat. BMC Plant Biol. 25 (2025) 987. https://dx.doi.org/10.1186/s12870-025-06914-y
Zam T, D’Auria J C, Jaisi A:
Advancements in biotechnological approaches for the production of high-value metabolites in Punica granatum. ACS Food Sci.Technol. 5 (2025) 2086-2097. https://dx.doi.org/10.1021/acsfoodscitech.5c00190
Chavez B G, Leite Dias S, DAuria J C:
The evolution of tropane alkaloids: Coca does it differently. Curr. Opin. Plant Biol. 81 (2024) 102606. https://dx.doi.org/10.1016/j.pbi.2024.102606
DAuria J C, Fernie A R:
The BAHD and the bold: the mitochondrias role in alkaloid artistry. Trends Plant Sci. 29 (2024) 1290-1291. https://dx.doi.org/10.1016/j.tplants.2024.07.012
Franke K, Stark P, Nagia M, Rizzo P, Wessjohann L A:
Metabolite profiling based characterization of active ingredients from St. Johns Wort. Z. Arznei Gewürzpfla. 28 (2024) 57-60.
Leite Dias S, Chuang L, Liu S, Seligmann B, Brendel F L, Chavez B G, Hoffie R E, Hoffie I, Kumlehn J, Bültemeier A, Wolf J, Herde M, Witte C-P, DAuria J C, Franke J:
Biosynthesis of the allelopathic alkaloid gramine in barley by a cryptic oxidative rearrangement. Science 383 (2024) 1448-1454. https://dx.doi.org/10.1126/science.adk6112
Naake T, DAuria J C, Fernie A R, Scossa F:
Phylogenomic and synteny analysis of BAHD and SCP/SCPL gene families reveal their evolutionary histories in plant specialized metabolism. Philos. Trans. R. Soc. Lond. B Biol. Sci. 379 (2024) 20230349. https://dx.doi.org/10.1098/rstb.2023.0349
Chavez B G, DAuria J C:
Turning a new leaf on cannabinoids. Nat. Plants 9 (2023) 687-688. https://dx.doi.org/10.1038/s41477-023-01415-y
Leite Dias S, Garibay-Hernández A, Brendel F L, Chavez B G, Brückner E, Mock H-P, Franke J, DAuria J C:
A new fluorescence detection method for tryptophan- and tyrosine-derived allelopathic compounds in barley and lupin. Plants 12 (2023) 1930. https://dx.doi.org/10.3390/plants12101930
Moghe G, Kruse L H, Petersen M, Scossa F, Fernie A R, Gaquere E, DAuria J C:
BAHD Company: The ever-expanding roles of the BAHD acyltransferase gene family in plants. Annu. Rev. Plant Biol. 74 (2023) 165-194. https://dx.doi.org/10.1146/annurev-arplant-062922-050122
Ravindran B M, Rizzo P, Franke K, Fuchs J, DAuria J C:
Simple and robust multiple shoot regeneration and root induction cycle from different explants of Hypericum perforatum L. genotypes. Plant Cell Tiss. Organ Cult. 152 (2023) 1-15. https://dx.doi.org/10.1007/s11240-022-02370-w
Rizzo P, Chavez B G, Leite Dias S, DAuria J C:
Plant synthetic biology: from inspiration to augmentation. Curr. Opin. Biotechnol. 79 (2023) 102857. https://dx.doi.org/10.1016/j.copbio.2022.102857
Sunic K, DAuria J C, Šarkanj B, Spanic V:
Metabolic profiling identifies changes in the winter wheat grains following Fusarium treatment at two locations in Croatia. Plants 12 (2023) 911. https://dx.doi.org/10.3390/plants12040911
Wang Y-J, Tain T, Yu J-Y, Li J, Xu B, Chen J, DAuria J C, Huang J-P, Huang S-X:
Genomic and structural basis for evolution of tropane alkaloid biosynthesis. Proc. Natl. Acad. Sci. U.S.A. 120 (2023) e2302448120. https://dx.doi.org/10.1073/pnas.2302448120
Brendel F:
Plant Wars: Gramine strikes back! A characterization and isolation of genes and enzymes involved in gramine biosynthesis from barley. (Bachelor Thesis) Halle/S., Martin-Luther-Universität Halle-Wittenberg (2022) 47 pp.
Chavez B G, Srinivasan P, Glockzin K, Kim N, Montero Estrada O, Jirschitzka J, Rowden G, Shao J, Meinhardt L, Smolke C D, DAuria J C:
Elucidation of tropane alkaloid biosynthesis in Erythroxylum coca using a microbial pathway discovery platform. Proc. Natl. Acad. Sci. U.S.A. 119 (2022) e2215372119. https://dx.doi.org/10.1073/pnas.2215372119
DAuria J C, Cohen S P, Leung J, Glockzin K, Glockzin K M, Gervay-Hague J, Zhang D, Meinhardt L W:
United States tea: A synopsis of ongoing tea research and solutions to United States tea production issues. Front. Plant Sci. 13 (2022) 934651. https://dx.doi.org/10.3389/fpls.2022.934651
Gippert A-L, Madritsch S, Woryna P, Otte S, Mayrhofer M, Eigner H, Garibay-Hernández A, DAuria J C, Molin E M, Mock H-P:
Unraveling metabolic patterns and molecular mechanisms underlying storability in sugar beet. BMC Plant Biol. 22 (2022) 430. https://dx.doi.org/10.1186/s12870-022-03784-6
Hall R D, DAuria J C, Silva Ferreira A C, Gibon Y, Kruszka D, Mishra P, van de Zedde R:
High-throughput plant phenotyping: a role for metabolomics? Trends Plant Sci. 27 (2022) 549-563. https://dx.doi.org/10.1016/j.tplants.2022.02.001
Saado I, Chia K-S, Betz R, Alcântara A, Pettkó-Szandtner A, Navarrete F, DAuria J C, Kolomiets M V, Melzer M, Feussner I, Djamei A:
Effector-mediated relocalization of a maize lipoxygenase protein triggers susceptibility to Ustilago maydis. Plant Cell 34 (2022) 2785–2805. https://dx.doi.org/10.1093/plcell/koac105
Alseekh S, Aharoni A, Brotman Y, Contrepois K, DAuria J C, Ewald J, Ewald J C, Fraser P D, Giavalisco P, Hall R D, Heinemann M, Link H, Luo J, Neumann S, Nielsen J, Perez de Souza L, Saito K, Sauer U, Schroeder F C, Schuster S, Siuzdak G, Skirycz A, Sumner L W, Snyder M P, Tang H, Tohge T, Wang Y, Wen W, Wu S, Xu G, Zamboni N, Fernie A R:
Mass spectrometry-based metabolomics: a guide for annotation, quantification and best reporting practices. Nat. Methods 18 (2021) 747-756. https://dx.doi.org/10.1038/s41592-021-01197-1
Fischer M:
Enzymatic decarboxylation of S-adenosylmethionine using S-adenosylmethionine decarboxylase. (Bachelor Thesis) Halle/S., Martin-Luther-Universität Halle-Wittenberg (2021) 39 pp.
Irfan M, Chavez B, Rizzo P, DAuria J C, Moghe G D:
Evolution-aided engineering of plant specialized metabolism. aBIOTECH 2 (2021) 240-263. https://dx.doi.org/10.1007/s42994-021-00052-3
Kim N, Chavez B, Stewart C, DAuria J C:
Structure and function of enzymes involved in the biosynthesis of tropane alkaloids. In: Srivastava V, Mehrotra S, Mishra S (Eds.): Tropane alkaloids – pathways, potential and biotechnological applications. Singapore: Springer Nature (2021) ISBN 978-981-334-534-8, 21-50.
DAuria J C (Ed.):
Special Issue: Advances in Plant Alkaloid Research; printed edition. (Series: Molecules, Vol. 25) (2020) ISBN 978-3-03943-172-4 (Hbk); ISBN 978-3-03943-173-1 (PDF), 278 pp. https://doi.org/10.3390/books978-3-03943-173-1
Isayenkov S, Hilo A, Rizzo P, Tandron Moya Y A, Rolletschek H, Borisjuk L, Radchuk V:
Adaptation strategies of halophytic barley Hordeum marinum spp marinum to high salinity and osmotic stress. Int. J. Mol. Sci. 21 (2020) 9019. https://dx.doi.org/10.3390/ijms21239019
Rizzo P, Altschmied L, Ravindran B M, Rutten T, DAuria J C:
The biochemical and genetic basis for the biosynthesis of bioactive compounds in Hypericum perforatum L., one of the largest medicinal crops in Europe. Genes 11 (2020) E1210. https://dx.doi.org/10.3390/genes11101210
Stark P, Zab C, Porzel A, Franke K, Rizzo P, Wessjohann L A:
PSYCHE – a valuable experiment in plant NMR-metabolomics. Molecules 25 (2020) 5125. https://dx.doi.org/10.3390/molecules25215125
Restrepo D A, Saenz E, Jara-Muñoz O A, Calixto-Botia I F, Rodríguez-Suárez S, Zuleta P, Chavez B G, Sanchez J A, DAuria J C:
Erythroxylum in focus: an interdisciplinary review of an overlooked genus. Molecules 24 (2019) 3788. https://dx.doi.org/10.3390/molecules24203788
scroll top